Chapter 14. Molecular Genetic Technology
Molecular Genetic Technology, or Biotechnology, is the modification of the living world around us for human benefit. There are different tools developed by biotechnologist over the last 70 years that allow scientists to modify the biological functions of living organisms at the molecular level. Techniques employed by molecular geneticists include cloning, stem cell manipulations, recombinant DNA technology, and gene editing. Biotechnology applications include gene therapy, bioremediation, Genetically Modified Organisms (GMOs), forensic science (DNA fingerprinting), and personal genomics (pharmacogenomics, genetic genealogy).
Cloning And Stem Cell Research
Cloning
The word “cloning” refers to making an identical copy of something (usually several times over).
Ex.: skin cells clone themselves through mitotic division.
Cloning, as applied to eukaryotic organisms, can be divided into three categories.
Gene cloning (producing recombinant DNA) is an insertion of a genetic sequence of interest into a vector (plasmid) and propagation of the resulting construct until a sufficient amount of inserted (recombinant) DNA is produced.
Reproductive cloning refers to a generation of a multicellular organism from a DNA sample from an oocyte carrying the nucleus from a somatic cell of the individual being cloned. The goal of reproductive cloning is to produce a viable individual genetically identical to the donor of the somatic nucleus (for example, cloning an entire sheep from an udder cell of that sheep).
Therapeutic cloning is the generation of embryonic or induced pluripotent stem cells (iPSCs) containing the donor’s DNA for subsequent growth of tissue for donor’s transplantation needs.
Therapeutic cloning is concerned with the propagation of cells of a particular individual for therapeutic purposes and not for reproductive purposes.
Therapeutic Cloning and Embryonic Stem Cells
The word “cloning” in this context refers to a process of initiation of embryonic cell division from a genetic material that replaces the genetic material of the ovum, thus by-passing the fertilization of an egg by a sperm. As such, cloning refers specifically to propagating of totipotent embryonic cells till the blastocyst stage of development. Consequently, there is no intent to actually clone a human being through this procedure. At the same time, since the use of embryonic stem cells has been surrounded by controversy, therapeutic cloning has recently taken advantage of the induced pluripotent stem cells (iPSCs) derived from adult multipotent stem cells.
The potency of stem cells
The potency of a cell specifies its differentiation potential, or potential to differentiate into different cell types. Totipotency is the ability of a single cell to divide and produce all the differentiated cells in an organism, including extraembryonic tissues. After reaching the 16-cell stage post-fertilization in a developing embryo, the totipotent cells of the morula differentiate into cells that will eventually become either the blastocyst’s Inner cell mass or the outer trophoblasts (see Chapter 13). Approximately four days after fertilization and after several cycles of cell division, these totipotent cells begin to specialize. The inner cell mass, the source of embryonic stem cells, is pluripotent, not totipotent.
Pluripotency (from the Latin plurimus, meaning very many, and potens, meaning having power) refers to a stem cell that has the potential to differentiate into any of the three germ layers: endoderm (interior stomach lining, gastrointestinal tract, the lungs), mesoderm (muscle, bone, blood, urogenital), or ectoderm (epidermal tissues and nervous system). Pluripotent stem cells can give rise to any fetal or adult cell type. However, alone they cannot develop into a fetal or adult organism because they, unlike the totipotent cells of the morula, lack the potential to contribute to extraembryonic tissue, such as the placenta.
Multipotent stem cells are a specialized cell type that is produced from pluripotent stem cells. They are found in adult tissues. A multipotent stem cell can give rise to other types of cells but it is limited in its ability to differentiate. These other types of cells are also limited in numbers. Examples of multipotent stem cells include those in the brain that give rise to different neural cells and glia or haematopoietic cells, which can give rise to different blood cell types, but they can’t create brain cells. Bone marrow also contains multipotent stem cells that give rise to all blood cell types but not other cells. Multipotent stem cells are essentially committed to produce specific cell types.
Induced Pluripotent Stem Cells (iPSCs)
Induced pluripotent stem cells (iPSCs) are derived from adult skin or blood stem cells or the fully differentiated somatic cells that have been reprogrammed back into an embryonic-like pluripotent state that enables the development of an unlimited source of any type of human cell needed for therapeutic purposes. For example, iPSC can be prodded into becoming beta islet cells to treat diabetes, blood cells to create new blood free of cancer cells for a leukemia patient, or neurons to treat neurological disorders.
Originally, iPSCs were expected to replace therapies based on the use of embryonic stem cells in regenerative medicine. The idea was to take a person’s skin, blood or other cells, reprogram them into iPS cells, and then use those to grow liver cells, neurons or whatever was needed to treat a disease. This personalized therapy would get around the risk of immune rejection as well as sidestepping the ethical concerns of using cells derived from embryos. However, with time the goals have shifted — in part because those therapies have proved challenging to develop. The only clinical trial using iPSC cells was halted in 2015 after just one person had received a treatment.
But iPS cells have made their mark in a different way. They have become an important tool for modelling and investigating human diseases, as well as for screening drugs. Improved ways of making the cells, along with gene-editing technologies, have turned iPSC cells into a lab workhorse — providing an unlimited supply of once-inaccessible human tissues for research.
This has been especially valuable in the fields of human development and neurological diseases. Nevertheless, a number of potentially therapeutic applications have been developed in the last few years, such as the production of neural stem cells from fibroblasts to fight glioblastoma tumors.
Could Skin Cells Be Used To Kill Cancer?
By Kathleen Lees
First Posted: Feb 24, 2016 06:42 PM EST
https://www.scienceworldreport.com/articles/37684/20160224/could-skin-cells-be-used-to-kill-cancer.htm
The reposting or the article adheres to the Fair Use application of 17 U.S. Code § 107, https://www.copyright.gov/title17/92chap1.html#107)
Researchers have discovered a way to turn skin cells into cells that fight cancer. Researchers at the University of North Carolina discovered this technique that fights remnant brain tumor cells in mice. In humans with glioblastomas–an aggressive form of brain cancer in which–only 30 percent patients survive past the two-year mark following a diagnosis.
“We wanted to find out if these induced neural stem cells would home in on cancer cells and whether they could be used to deliver a therapeutic agent,” Dr. Shawn Hingtgen, an assistant professor at the University of North Carolina, said in a news release. “This is the first time this direct reprogramming technology has been used to treat cancer.” During the study, researchers reprogrammed fibroblasts–otherwise known as skin cells- -in order to make them neural stem cells and create a tumor-killing protein. The cells, which in skin produce collagen and connective tissue, hunt and kill cancer cells, according to UPI.
Based on the type of tumor, researchers were able to increase survival time in the mice from 160 to 220 percent. With future studies, researchers hope to focus on human stem cells and testing more effective anti-cancer drugs that can be loaded into tumor-seeking neural stem cells.
“Our work represents the newest evolution of the stem-cell technology that won the Nobel Prize in 2012,” Hingtgen said, via Medical Xpress. “We wanted to find out if these induced neural stem cells would home in on cancer cells and whether they could be used to deliver a therapeutic agent. This is the first time this direct reprogramming technology has been used to treat cancer.” The findings are published in the journal Nature Communications.
Recombinant DNA Technology
Recombinant DNA (rDNA) molecules are DNA sequences that result from the use of laboratory methods (molecular cloning) to bring together genetic material from multiple sources, creating sequences that would not otherwise be found in biological organisms. Recombinant DNA is possible because DNA molecules from all organisms share the same chemical structure; they differ only in the sequence of nucleotides within that identical overall structure. Consequently, when DNA from a foreign source is linked to host sequences that can drive DNA replication and then introduced into a host organism, the foreign DNA is replicated along with the host DNA.
Cloning vectors
Formation of recombinant DNA requires a cloning vector, a DNA sequence cabable of carrying DNA from another source that can replicate within a living cell. Vectors are generally derived from plasmids or viruses.
A plasmid is a small DNA molecule that is physically separate from, and can replicate independently of, chromosomal DNA within a cell. Most commonly found as small circular, double-stranded DNA molecules in bacteria, plasmids are sometimes present in archaea and eukaryotic organisms. In nature, plasmids carry genes that may benefit survival of the organism (e.g., antibiotic resistance), and can frequently be transmitted from one bacterium to another, even of another species. Artificial plasmids are widely used as vectors in molecular cloning, serving to drive the replication of recombinant DNA sequences within host organisms.
Restriction Enzymes
DNA restriction endonucleases, or restriction enzymes, recognize short, specific
palindrome sequences of DNA double-stranded nucleotides and make breaks in the sugar- phosphate backbone of the DNA in the region of the recognized sequence.
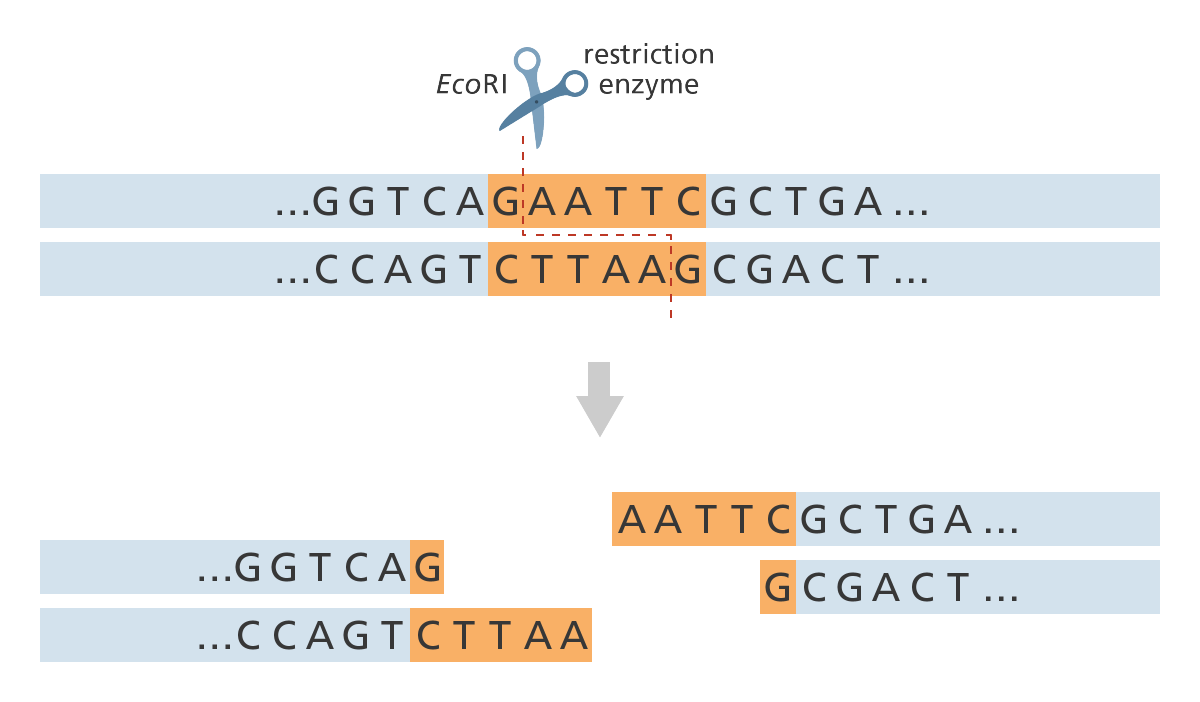
Restriction enzymes, also called restriction nucleases) surround the DNA molecule at the recognized sequence (GAATTC for EcoRI). They cut one strand of the DNA double helix at one point and the second strand at a different, complementary point (for EcoRI, between the G and the A base).
DNA micromanipulations: Recombinant plasmids and DNA cloning
The use of cloning is interrelated with recombinant DNA in classical biology, as the term “clone” refers to a cell or organism derived from a parental organism, with modern biology referring to the term as a collection of cells derived from the same cell that remain identical. In the classical instance, the use of recombinant DNA provides the initial cell from which the host organism is then expected to recapitulate when it undergoes further cell division, with bacteria remaining a prime example due to the use of viral vectors in medicine that contain recombinant DNA inserted into a structure known as a plasmid.
Plasmids are extrachromosomal self-replicating circular forms of DNA present in most bacteria, such as Escherichia coli (E. coli), containing genes related to catabolism and metabolic activity, and allowing the carrier bacterium to survive and reproduce in adverse environments. Genes carried by plasmids include those of antibiotic resistance. Plasmids’ DNA is usually several thousand base pairs in size, and can be modified by extracting them from the host bacterium, and inserting or removing genetic sequences with the use of restriction endonucleases. By accepting a DNA sequence of interest, a plasmid becomes a cloning vector, which can be re-inserted back into a bacterial cell, which would then be allowed to multiply, and, thus, to increase the copy number of the recombinant DNA plasmids they carry. The size of a genetic sequence a plasmid can accept is limited, and better integration rates are usually achieved with fragments comparable with the plasmid’s own size. For larger genetic sequences, alternative vectors, such as phagemids or artificial bacterial or yeast chromosomes (BACs and YACs), are used.
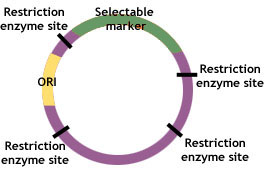
Plasmids contain three common genetic features—a replicator, a selectable marker and a cloning site. The replicator or “ori” refers to the origin of replication of plasmid’s DNA. The marker refers to a particular gene that usually contains resistance to an antibiotic, but may also be a gene that is attached alongside the desired one, such as that which confers luminescence to allow identification of successfully recombined DNA. The cloning site is a sequence of nucleotides representing one or more posi- tions where cleavage by restriction endonucleases occurs.
Biotechnology and human disease: manufacturing insulin
Eukaryotic genes can also be artificially expressed in prokaryotic cells (E. coli) by a physical transfer into a prokaryotic genome. Prokaryotic cells expressing eukaryotic genes can produce large amounts of eukaryotic protein like insulin.
Biotechnology is the manipulation of biological systems to manufacture important foods, drugs, biopolymers and other materials. This is illustrated below by the production of the protein human insulin in bacterial culture.
1. A recombinant DNA plasmid is produced to include the piece of human DNA containing instructions for making proinsulin. Proinsulin is the precursor for insulin.
2. The plasmid is then inserted into E. coli bacteria, which can now produce human proinsulin, along with bacterial own proteins.
3. The bacteria multiply and produce large quantities of human proinsulin in a fermentor.
4. Bacteria are then killed by heat sterilization, leaving behind the proinsulin.
5. Proinsulin is enzymatically cleaved to produce human insulin. Enzymatic cleavage is the slicing of a molecule by an enzyme.
6. The solution is centrifuged to remove cell matter and purified by liquid chromatography and crystallization.
Polymerase Chain Reaction (PCR)
Polymerase chain reaction (PCR) is a form of gene cloning that does not involve plasmids. PCR is a technique to amplify a single or a few copies of a piece of DNA across several orders of magnitude, generating thousands to millions of copies of a particular DNA sequence. The method relies on thermal cycling, consisting of cycles of repeated heating and cooling of the reaction for DNA denaturation and re-annealing and enzymatic replication of the DNA, using a thermostable DNA polymerase.
PCR reaction components include DNA template that contains the DNA region (tar- get) to be amplified; two primers that are complementary to the 3’ ends of each of the strand of the DNA target; DNA polymerase with a temperature optimum at around 70 °C, and deoxynucleoside triphosphates (dNTPs), the building blocks from which the DNA polymerases synthesizes a new DNA strand. A PCR reaction also needs to be supplied with a buffer solution, providing a suitable chemical environment for optimum activity and stability of the DNA polymerase. Divalent cations, such as magnesium or manganese ions, are important polymerase co-factors and need to be supplied in the buffer as well.
Quantitative PCR (qPCR)
Quantitative PCR (qPCR) is a PCR technique that allows researchers to estimate the quantity of starting material in a DNA sample. Please watch the following Youtube video for additional details about the qPCR technique and its application in detecting the presence of COVID-19 virus in a biological sample.
DNA sequencing
Sanger dideoxynucleotide terminator-cycle sequencing
In 1977, Frederick Sanger devised the chain-termination dideoxynucleotide method for sequencing. The fluorescent dye-terminator cycle sequencing method, adapted for PCR amplification in 1980s, is based on this concept.
The PCR reaction includes the following ingredients: DNA template, primer, Taq DNA polymerase, unlabeled dNTP’s, fluorescently labeled ddNTP’s, buffer, and distilled water. The fluorescently labeled ddNTP’s are called dideoxynucleotides, named so because they lack a 3′ OH. Due to that chemical modification, no additional nucleotides can be added to the chain once a ddNTP has been added; therefore, the sequence terminates in a fluorescently labeled ddNTP. Each base has a differently col- ored dye that can be recognized by a laser.
During PCR, the temperature is increased to ~95 degrees Celsius to denature the double-stranded DNA. Then the temperature drops to ~50 degrees Celsius to allow the primers to anneal to the template. Finally, Taq polymerase adds nucleotides to the growing chain at ~60 degrees Celcius. This cycle repeats 25-30 times. During the last step, the Taq polymerase adds ei- ther the dNTP’s or the ddNTP’s to the chain randomly. Therefore, when PCR is complete, you are left with PCR fragments of all different lengths; for whenever a ddNTP was added, the elongation stopped.
You can then load the entire PCR reaction into one lane of a polyacrylamine gel in a special laser sequencer. The sequencer can read the different dyes as they pass the laser at the bottom of the gel. Because the different fragments migrate toward the positively-charged pole at different rates, the shorter fragments migrate toward the bottom of the gel faster, so you read it bottom->top (5′->3′). The computer then compiles these data into the image of a gel. The colors are then read as bases to produce a chromatogram of the piece of DNA sequenced. The sequence can be taken right from the chromatogram with an extremely high level of accuracy.
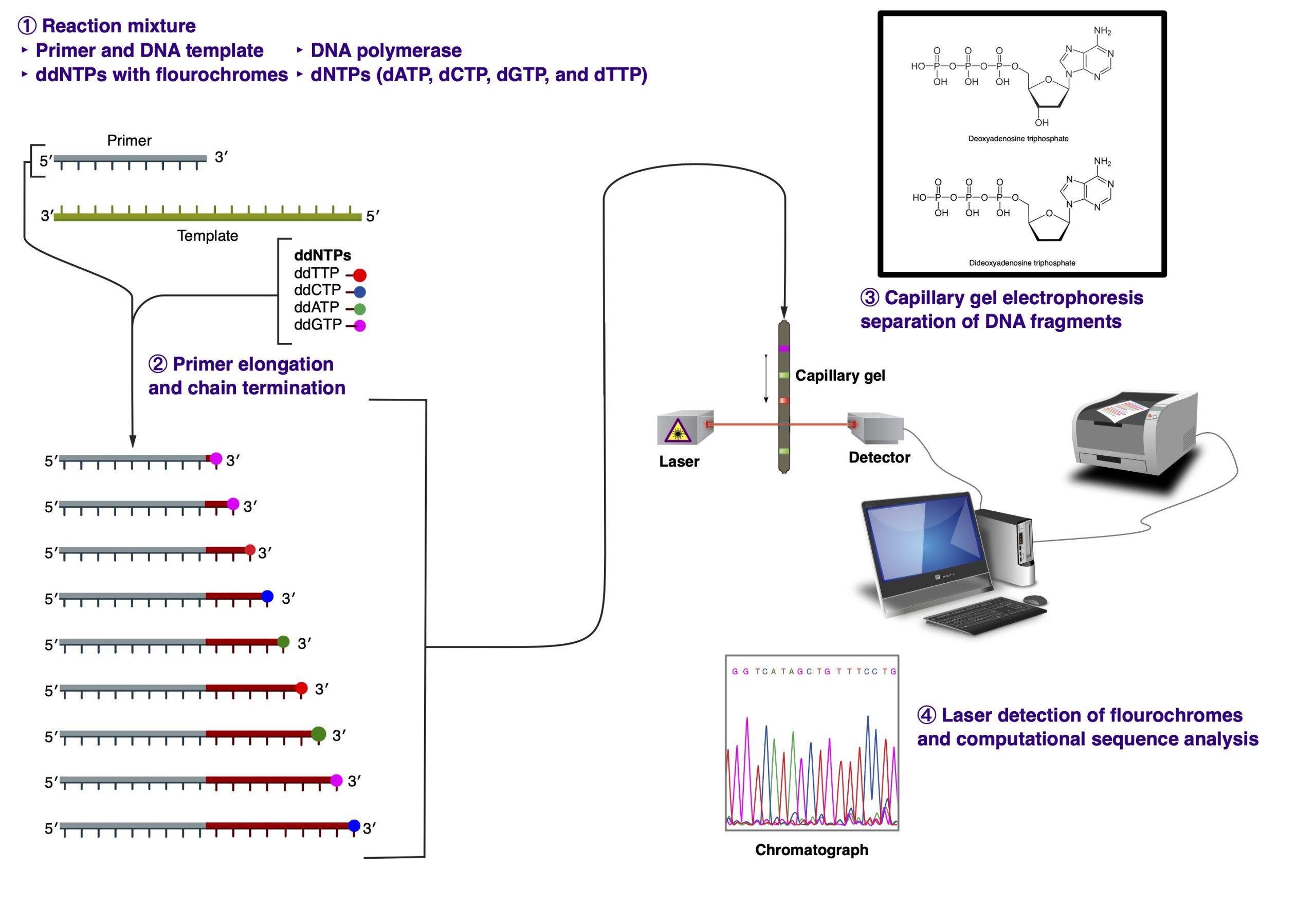
The main limitation of the Sanger sequencing method is in the size of the DNA fragments that can be sequenced. Typically, the best reading resolution is achieved for sequences 100 to 300 nucleotides in length (excluding primer sequences). It is not uncommon to get a decent readout for sequences in the 500 nucleotide range, but the equality of the sequencing read starts to drop precipitously after that.
Next generation sequencing (NGS)
Next generation sequencing (NGS) is often referred to as massively parallel sequencing, which means that millions of small fragments of DNA can be sequenced at the same time, creating a massive pool of data. This pool of data can reach gigabites in size, which is the equivalent of 1 billion (1,000,000,000) base pairs of DNA. In comparison, previous methods could sequence one DNA fragment at a time, perhaps generating 500 to 1000 base pairs of DNA in a single reaction.
‘Traditional’ genetic testing involved the sequence analysis of a number of genes in a step-wise manner to try to identify where a genetic alteration may be. An example of this could be the genetic testing involved in cases of inherited breast cancer where two genes, BRCA1 and BRCA2, are usually sequenced in the laboratory to look for genetic alterations in affected patients. This could require sequencing of more than 60 fragments or pieces of DNA to identify where a single DNA base change may be present. Using NGS these genes (and perhaps others that have potential clinical relevance) could be sequenced all at once in a single test. This clearly has the potential to provide information on lots of genes all at once.
Another example might be where there are lots of genes involved in the development of a tumor such as lung cancer. Looking at the molecular profile (or genetic make-up) of the tumor sample using NGS might lead to the identification of an alteration that could suggest the best drug treatment option for a patient or perhaps a clinical trial that the patient could be entered into in the development of new and effective cancer treatments. This is in the area of stratified medicine.
Extending this technology further, it is becoming possible to undertake whole exome or genome analysis for patients. This is a particular area that would not have been possible without the development of NGS. In this type of analysis, it is possible for scientists to look at the entire genetic make-up of a patient. The genome is all of the genetic material in an individual and includes all of the genes and DNA that each cell in the human body contains.
This is an incredibly powerful development, and whilst offering the potential to identify exactly where a gene alteration might be present in a patient, it does also provide the potential to identify some unexpected findings as well. Consequently this testing is being used very carefully and with the full consent and under- standing of the patient.
The introduction of NGS also has a significant part to play in the area of genomics. This is particularly relevant in stratified or per- sonalized medicine where it is possible to identify the genetic alterations that can predict how a patient might respond to a specific drug and decide what dose might be most effective. To achieve this it is necessary to ‘profile’ or sequence a number of genes to identify the most relevant genetic alterations.
Perhaps the biggest challenge for the genetics laboratory when creating large amounts of sequence data by NGS is the vast quantity of computer data that is produced. This can be as much as 600 gigabytes in a single run. That is the equivalent of perhaps 90,000 songs on an MP3 player. Bearing in mind this is data from a single NGS run, then over the weeks, months and years NGS will produce large amounts of data that require management and storage in a safe and secure manner. In addition there is significant reviewing and scientific interpretation of the data produced to translate into useful information that can be passed back to the clinician and patient.
Shotgun DNA Sequencing
Shotgun DNA sequencing refers to the NGS technique where researchers first shred DNA into small fragments, then sequence the fragments, followed by arranging the fragments in one linear sequence to reassemble the entire genome. Please read more about Shotgun DNA Sequencing under the following link:
https://www.genome.gov/genetics-glossary/Shotgun-Sequencing
Exome Sequencing
Exome Sequencing targets the protein-coding portion of the genome for sequencing. Protein-coding regions comprise about 2.5% of the human genome (see Chapter 1), which in reality amounts to a little over 1% of we consider just the exon (protein-coding) portion of the protein-coding DNA (excluding introns and various control sequences). The collection of all exon sequences in the genome is called an exome. At the same time, the exome harbors 85% of all disease-causing mutations in human DNA. For clinical purposes, exome sequencing is a more targeted and more cost-effective alternative to the whole genome sequencing.
Gene editing
CRISPRs (clustered regularly interspaced short palindromic repeats) are segments of prokaryotic DNA containing short repetitions of base sequences. The CRISPR/Cas system is a prokaryotic immune system that confers resistance to foreign genetic elements such as plasmids and phages. The CRISPR/Cas system has been used for gene editing (adding, disrupting or changing the sequence of specific genes) and gene regulation. CRISPR/Cas is a radically fast and easy way to precisely cut and replace DNA sections in a living organism.
CRISPR/Cas DNA editing system consists of two components: a “guide” RNA (gRNA) and a non-specific CRISPR-associated endonuclease (Cas9). The gRNA is a short synthetic RNA composed of a “scaffold” sequence necessary for Cas9-binding and a user-defined ∼20 nucleotide “spacer” or “targeting” sequence which defines the genomic target to be modified. Thus, one can change the genomic target of Cas9 by simply changing the tar- geting sequence present in the gRNA.
Gene therapy
Gene therapy involves the transfer of a therapeutic or working gene copy into specific cells of an individual. It may be used to:
- Replace a faulty gene
- Introduce a new gene whose function is to cure or to fa- vorably modify the clinical course of a condition
- Inactivate or “knock out” a faulty gene that is not functioning properly.
The potential of gene therapy is very broad, with research involving a number of diseases such as severe combined immune-deficiencies (SCID), hemophilia, Parkinson’s disease, many forms of cancer and HIV.
There are many challenges to successful gene therapy.
First, the condition in question must be well understood and the underlying causative gene identified. A working copy of the gene involved must be available and the specific cells in the body requiring treatment must be identified, accessible and a means of efficiently delivering working copies of the gene to these cells must be available.
Of all these challenges, the one that is most difficult is the problem of gene delivery, i.e. how to get the new or replacement genes into the desired tissues.
One of the most promising methods currently being developed is the use of harmless viruses that can be used to carry genes into cells. Scientists now have the knowledge and skills to remove the virus’ own genes and to replace them with working human genes. These altered viruses can then be used to deliver genes into cells with great efficiency. When viruses are used in this way they are known as vectors.
Some of these vectors are capable of not only carrying the gene into the cell but also of inserting the gene into the genetic material of the cell. Once in the right location within the cell of an affected person, the transplanted gene is switched on. The transplanted gene can then issue the instructions necessary for the cell to make the protein that was previously missing or altered.
Another technique with a promising potential is the use of stem cells in delivering gene therapy. Stem cells are cells that have not yet differentiated into a specific tissue or organ cell.
In this technique, stem cells are manipulated in the laboratory in order to make them accept new genes that can then change their behavior. For example, a gene might be inserted into a stem cell that could make it better able to survive chemotherapy. This would be of assistance to those patients who could benefit from further chemotherapy following stem cell transplantation.
Applications of Molecular DNA Technology in Forensic Science and Human Identity Research
Forensic DNA analysis utilizes genetic tools and scientific methodology to examine biological evidence in the process of criminal and civil litigation. Human identity analysis is used in broader applications, such as ancestry analysis.
The techniques used by forensic and identity DNA analysis have vastly evolved in the recent decades. Depending on the information needed, there are several different techniques that can be used to type a DNA fragment. Short tandem repeat (STR) fragment analysis, Sanger sequencing followed by capillary electrophoresis are some of the techniques that have been used for the genetic analysis of DNA samples. NGS is the newest and most revolutionary technology that is becoming a commonplace in the genetic analysis of forensic and identity samples.
The Ubiquity of Human DNA in the Environment
Molecular genetic technology can be used to identify individuals using the DNA they leave behind. Humans “shed” their DNA anywhere they go. Human DNA can be found in the soil, water, and air. While the ability to extract and analyze DNA from environment using eDNA techniques can be a useful tool in tracking down people who break the law, it can also be used for less legally justifiable purposes.
New threat to privacy? Scientists sound alarm about DNA tool
By Juliette Collen, AFP
Mon, May 15, 2023
https://news.yahoo.com/threat-privacy-scientists-sound-alarm-150315334.html
The reposting of the article adheres to the Fair Use application of 17 U.S. Code § 107 – Limitations on Exclusive Rights: Fair Use, https://www.copyright.gov/title17/92chap1.html#107)
The traces of genetic material that humans constantly shed wherever they go could soon be used to track individual people, or even whole ethnic groups, scientists said on Monday, warning of a looming “ethical quagmire”.
A recently developed technique can glean a huge amount of information from tiny samples of genetic material called environmental DNA, or eDNA, that humans and animals leave behind everywhere — including in the air.
The tool could lead to a range of medical and scientific advances, and could even help track down criminals, according to the authors of a new study published in the journal Nature Ecology & Evolution.
But it also poses a vast range of concerns around consent, privacy and surveillance, they added.
Humans spread their DNA — which carries genetic information specific to each person — everywhere, by shedding skin or hair cells, coughing out droplets, or in wastewater flushed down toilets.
In recent years, scientists have been increasingly collecting the eDNA of wild animals, in the hopes of helping threatened species.
For the new research, scientists at the University of Florida’s Whitney Laboratory for Marine Bioscience had been focused on collecting the eDNA of endangered sea turtles.
– ‘Human genetic bycatch’ –
But the international team of researchers inadvertently collected a massive amount of human eDNA, which they called “human genetic bycatch”.
David Duffy, a wildlife disease genomic professor at the Whitney Laboratory who led the project, said they were “consistently surprised” by the amount and quality of the human eDNA they collected.
“In most cases the quality is almost equivalent to if you took a sample from a person,” he said.
The scientists collected human eDNA from nearby oceans, rivers and towns, as well as from areas far from human settlements.
Struggling to find a sample not tainted by humans, they went to a section of a remote Florida island inaccessible to the public.
It was free of human DNA — at least until a member of the team walked barefoot along the beach. They were then able to detect eDNA from a single footprint in the sand.
In Duffy’s native Ireland, the team found human DNA all along a river, with the exception of the remote mountain stream at its source.
Taking samples from the air of a veterinary hospital, the team captured eDNA that matched the staff, their animal patient and viruses common in animals.
– ‘Perpetual genetic surveillance’? –
One of the study’s authors, Mark McCauley of the Whitney Laboratory, said that by sequencing the DNA samples, the team was able to identify if a person had a greater risk of diseases such as autism and diabetes.
“All of this very personal, ancestral and health-related data is freely available in the environment, and it’s simply floating around us in the air right now,” McCauley told an online press conference.
“We specifically did not examine our sequences in a way that we would be able to pick out specific individuals because of the ethical issues,” he said.
But that would “definitely” be possible in the future, he added.
“The question is how long it takes until we’re at that stage.”
The researchers emphasised the potential benefits of collecting human eDNA, such as tracking cancer mutations in wastewater, discovering long-hidden archaeological sites or revealing the true culprit of a crime using only the DNA they left in a room.
Natalie Ram, a law professor at the University of Maryland not involved in the research, said the findings “should raise serious concern about genetic privacy and the appropriate limits of policing”.
“Exploiting involuntarily shed genetic information for investigative aims risks putting all of us under perpetual genetic surveillance,” she wrote in a commentary on the study.
The authors of the study shared her concerns.
McCauley warned harvesting human eDNA without consent could be used to track individual people or even target “vulnerable populations or ethnic minorities”.
It is why the team decided to sound the alarm, they said in a statement, calling for policymakers and scientists to start working on regulation that could address the “ethical quagmire”.
The article by Liam Whitmore et al., “Inadvertent human genomic bycatch and intentional capture raise beneficial applications and ethical concerns with environmental DNA”, can be accessed following this link: https://www.nature.com/articles/s41559-023-02056-2
Forensic DNA analysis (DNA fingerprinting)
Forensic science employs DNA analysis to identify individuals by their DNA profiles. Most commonly used forensic DNA approaches include the nuclear genome profile based on variable DNA repeats (VNTRs), Y-chromosome and mtDNA analysis.
Although 99.9% of human DNA sequences are the same in every person, enough of the DNA is different that it is possible to distinguish one individual from another, unless they are monozygotic (“identical”) twins. DNA profiling uses repetitive sequences that are highly variable, called variable number tandem repeats (VNTRs), in particular short tandem repeats (STRs). STRs are highly polymorphic regions that have short repeated sequences of DNA (the most common is 4 bases repeated, but there are other lengths in use, including 3 and 5 bases). VNTR loci are very similar between closely related humans, but are so variable that unrelated individuals are extremely unlikely to have the same VNTRs.
The system of DNA profiling used today is based on PCR and uses STRs. Because unrelated people almost certainly have different numbers of repeat units, STRs can be used to discriminate between unrelated individuals. These STR loci (locations on a chromosome) are targeted with sequence-specific primers and amplified using PCR. The DNA fragments that result are then separated and detected using electrophoresis.
In the US, the STR-based DNA profiling system screens for 13 polymorphic loci to establish a unique individual DNA profile.
The Combined DNA Index System (CODIS), a DNA database funded by the FBI, stores DNA profiles created by federal, state, and local crime laboratories in the United States, with the ability to search the database to assist in the identification of suspects in crimes.
Y-chromosome analysis
Recent innovations have included the creation of primers targeting polymorphic regions on the Y-chromosome (Y-STR), which allows resolution of a mixed DNA sample from a male and female or cases in which a differential extraction is not possible. Y-chromosomes are paternally inherited, so Y-STR analysis can help in the identification of paternally related males.
Mitochondrial analysis
For highly degraded samples, it is sometimes impossible to get a complete profile of the 13 CODIS STRs. In these situations, mitochondrial DNA (mtDNA) is sometimes typed due to there being many copies of mtDNA in a cell, while there may only be one to two copies of the nuclear DNA. Forensic scientists am- plify the region that controls replication of mtDNA (HV1 and HV2), and then sequence each region and compare single- nucleotide differences to a reference. Because mtDNA is maternally inherited, directly linked maternal relatives can be used as match references, such as one’s maternal grandmother’s daughter’s son. MtDNA is useful in determining clear identities, such as those of missing people when a maternally linked relative can be found. mtDNA testing was used in determining that Anna Anderson was not the Russian princess she had claimed to be, Anastasia Romanov. MtDNA can be obtained from such material as hair shafts and old bones/teeth.
Key Takeaways
- Recombinant DNA technology creates new genetic combinations that can be used for human benefit.
- A variety of genetic and genomic techniques developed in the last 50 years produce tools of discovery and analysis that may lead to purposeful alteration of any genetic sequence.
