Chapter 11. Chromosomal Abnormalities
The Spectrum of Human Genetic Disorders
Human diseases consist of those which are predominantly acquired (environmental, such as bacterial infections), multifactorial (have both a genetic and an environmental component), and those which are mostly genetic.
At the chromosomal level, human genetic disorders can result from changes in chromosomal number and chromosomal structure. The former are caused by mistakes in cell division and the resulting unequal distribution of the chromosomes to newly formed cells. The latter result from a diverse array of structural modifications cased by a number of factors and the majority occurring during crossing over in meiosis. Transposable elements play a significant role in chromosomal structural abnormalities.
Around the world, the prevalence of chromosomal abnormalities is about 0.4% (4/1000 live births, or 1 in 250). Of these, autosomal chromosomal abnormalities occur in 1 out of 264 live births. The most frequent chromosomal abnormality is Trisomy 21 (Down Syndrome, 1 in 361 live births).
Changes In Chromosomal Number
Cells with normal chromosome sets (two sets of 23 chromosomes in humans) have euploid (normal) karyotype. A change in normal chromosomal number involving single chromosomes within a diploid set leads to an aneuploid karyotype. An aneuploid karyotype is observed in 1 out of 250 live births in humans. Aneuploid individuals possess a genetic imbalance. Aneuploidy can occur in the result of either meiotic or mitotic non-disjunction.
Most chromosomal abnormalities lead to changes in the phenotype that are not compatible with life. Any polyploidy (extra copies of all chromosomes, i.e., triploidy, tertaploidy, etc.) higher than diploid results in early termination of pregnancy in utero. For example, triploidy (69, XXX or 69, XXY or 69, XYY) occurs in the result of double fertilization of an ovum (dispermy). The extra set of paternal chromosomes predisposes to formation of a partial mole, features of which may or may not be grossly or microscopically apparent.
Extra copies of a single chromosome (i.e., chromosome 21) is referred to as trisomy. Trisomy, except for chromosomes 13, 18, or 21, is not compatible with life. Trisomy 13 and Trisomy 18 each lead to early death, usually in the neonatal period of development, if not in utero.
Mechanisms of aneuploidy: Meiotic and mitotic nondisjunction
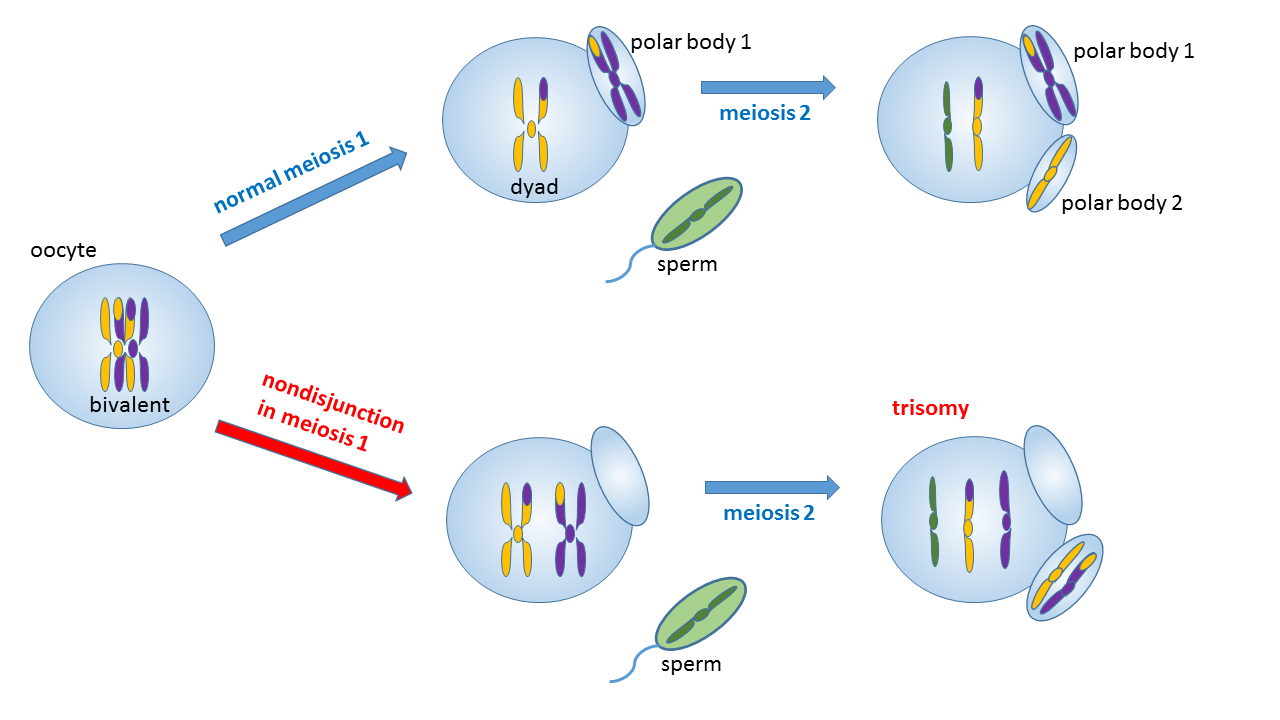
Nondisjunction (“not coming apart”) is the failure of chromosome pairs to separate properly during cell division. This could arise from a failure of homologous chromosomes to separate in Meiosis I, or the failure of sister chromatids to separate during Meiosis II or Mitosis. The result of this error is a cell with an imbalance of chromosomes.
In meiotic nondisjunction during Meiosis I, a pair of duplicated chromosomes (a tetrade on the Metaphase I plate) fails to separate in subsequent stages of Meiosis I division, resulting in the products of Meiosis I both being aneuploid (one resulting cell has one extra chromosome and the other one lacks a chromosome). In Meiosis II division, the aneuploid products of Meiosis I each produce two aneuploid gametes, two with an extra chromosome and two with a missing chromosome.
Credit: Wpeissner, CC BY-SA 3.0, via Wikimedia Commons
In a nondisjunction event during Meiosis II, one pair of sister chromatids in one of the two cells produced in Meiosis I fails to separate after Metaphase II, leading to the resulting products of that cell’s division being aneuploid (one having one less chromosome and one having an extra chromosome).
Thus, all four products of a meiotic cell division experiencing a chromosomal non-disjunction event during Meiosis I will be aneuploid, while only two out of four resulting meiotic products of a cell affected by a non-disjunction event during Meiosis II will be aneuploid. A fertilization event involving a gamete with normal haploid chromosomal complement and an aneuploid gamete will result in an aneuploid embryo.
Meiotic nondisjunction in either the first or second meiotic division is the major cause of chromosomal aneuploidy. The greatest percentage of these (75%) occurs in oogenesis, where the probability of non-disjunction increases with maternal age. Almost 80% occur in the first meiotic division. Meiotic nondisjunction may affect as many as 25% of all ova and 2% of all sperm.
Nondisjunction of sex chromosomes is better tolerated than non-disjunction of autosomes. An excess of chromosomes is tolerated better than a deficiency.
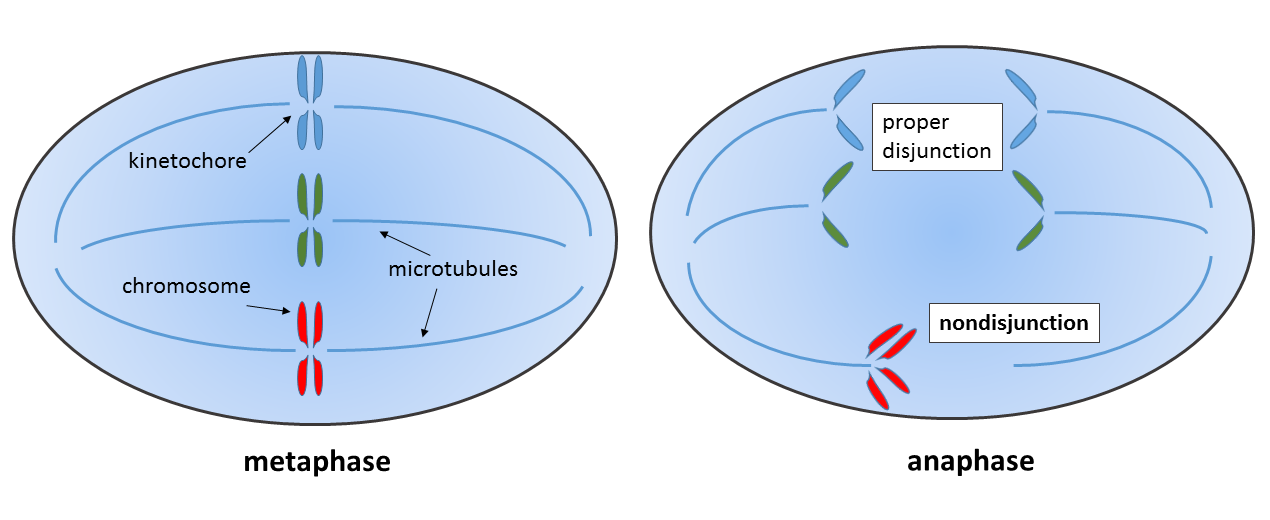
Mitotic nondisjunction occurs when two sister chromatids fail to separate during somatic cell division (see an example in the figure below). Mitotic non-disjunction can happen during development, but it also known to be associated with cancer.
Credit: Wpeissner, CC BY-SA 3.0, via Wikimedia Commons.
An individual with a mixture of cells with different chromosome number is called a mosaic. If two independent cell lines (from two zygotes) fuse early in development, such an individual will be referred to as a chimera. If the somatic nondisjunction occurred early in development, it may lead to germinal mosaicism.
Age-dependent aneuploidy in human oocytes is traced to defective linkages between sister chromatids in meiosis
Aneuploidy in human oocytes plays a significant role in causing infertility, miscarriage, and chromosomal disorders. The incidence of these aneuploidies rises substantially as women age, often attributed to faulty linkages between sister chromatids (called cohesion) during meiosis. A recent study suggests that the loss of a specific group of the cohesin protector protein, shugoshin 2 (SGO2), might be a contributing factor to this event. In the study, it was shown that SGO2 plays a crucial role in maintaining sister chromatid cohesion during meiosis by safeguarding a “cohesin bridge” connecting the sister chromatids. In human oocytes, SGO2 is present in both sub-centromere cups and the pericentromeric bridge, spanning the junction of sister chromatids. While SGO2 normally colocalizes with cohesin, in oocytes from older women, SGO2 is frequently absent from the pericentromeric bridge in Meiosis II , resulting in weakened sister chromatid cohesion. The kinase activities of MPS1 and BUB1 are responsible for maintaining SGO2 at sub-centromeres and the pericentromeric bridge. Inhibiting MPS1 during meiosis I diminishes SGO2, reducing cohesion protection and increasing the occurrence of single chromatids in Meiosis II. Consequently, SGO2 deficiency in human oocytes can compound the effects of maternal age by making the residual cohesin at pericentromeres susceptible to loss during anaphase I. Research demonstrates that compromised SGO2 localization affects cohesion integrity, potentially contributing to the age-dependent elevated incidence of aneuploidy observed in human oocytes.
Article link: Bettina P. Mihalas BP et al. (2024) Age-dependent loss of cohesion protection in human oocytes. Current Biology 34(1): 117-131.e5, https://doi.org/10.1016/j.cub.2023.11.061
Nondisjunction Of Autosomes
Nondisjunction of autosomes leads to extra or missing chromosomes. As a rule, autosomal nondisjunction in not tolerated. Only three autosomal aneuploids survive after birth in humans and all three are trisomies.
Trisomy 21 (47, XX/XY, +21)
Trisomy 21 is dubbed Down syndrome after the superintendent of an asylum for children with mental retardation in Rurrey, England, named John Langdon Down, who first studied the condition and published an essay on it in 1866.
There are two types of trisomy 21. One results from chromosomal nondisjunction, and another one is produced by a chromosomal translocation and is an inherited form on trisomy 21 (to be discussed in the next section).
The type of trisomy 21 caused by a nondisjunction cannot be inherited. People with this condition are not able to reproduce. Around 80% of all cases of trisomy 21 are caused by a non- disjunction during the first meiotic division of oogenesis, which shows strong maternal-age association. Maternal meiosis II errors constitute 20 to 24% of maternal errors and 18 to 20% of all cases of non-inherited trisomy 21 (see Robertsonian translocations further in the chapter for the inherited type of Down syndrome).
Trisomy 18: Edward’s Syndrome (47, XX/XY +18)
Trisomy 18 results mostly from maternal nondisjunction in meiosis II (during ovulation).
Frequency: 1/6,000 live births. 80% are XX; nearly all affected individuals die in their first year. Features include micrognathia (small lower jaw), overlapping fingers, horseshoe kidney, rocker bottom feet, cardiac defects, diaphragmatic hernia, omphalocele (defect in muscles of the abdominal wall).
Trisomy 13: Patau’s Syndrome (47, XX/XY +13)
Frequency: 1/12,000 live births; mean survival time is 3 months.
Features include microcephaly (small head), cleft lip and/or palate, postaxial polydactyly, cardiac defects, deafness, holoprosencephaly (malformation of the cerebrum).
Trisomy 16
Trisomy 16 is one of the most common chromosomal abnormalities, but the affected embryos or fetuses never survive past the first trimester. Many first trimester fetuses are lost in this fashion (many are “silent” miscarriages).
Nondisjunction Of Sex Chromosomes
Sex-chromosome abnormalities may also be caused by nondisjunction of one or more sex chromosomes. The majority of sex-chromosomal aneuploidies carry extra chromosomes. Only one sex chromosomal aneuploidy is a monosomy.
Multiple (up to four) X chromosomes are known to survive. Individuals with more than one X and a Y chromosome are usually underdeveloped and sterile.
XXX and XO individuals are known. XXX individuals produce oocytes and are fertile, although, in most cases, XO individuals have streak gonads and are sterile.
Klinefelter’s Syndrome (KS) (47, XXY)
Frequency: 1 in 650 newborns.
Klinefelter’s Syndrome manifests in individuals who have two to four X chromosomes resulting in the XXY sex chromosome composition. An extra Y chromosome may be present on some occasions giving the XXYY karyotype.
Conditions associated with KS include eunuchoid (tall) body proportions, sterility due to azoospermia (failure to produce sperm). Gynaecomastia (swollen breasts) is another condition characteristic to KS. Breast cancer is estimated to affect some 3% of KS individuals, about 20 times what one would expect to find in 46,XY individuals. Individuals with KS often present with a spectrum of psychiatric disorders. Respiratory disorders such as asthma seem to be much more common in individuals with KS.
Jacobs Syndrome (47, XYY)
Jacobs’ Syndrome frequency is estimated to be 1 per 1000 XY individuals, but is likely ten times higher due to a lack of a characteristic phenotype, leading to most of XYY individuals to be never diagnosed.
Jacobs syndrome is a chromosomal condition in which a person has an extra Y chromosome. Individuals with Jacobs are usually taller than average, but the person is otherwise phenotypically normal. XYY individuals may exhibit cognitive delays, the feature shared with XXX individuals, which can be avoided with early diagnosis. Early studies performed by psychiatrists on institutionalized persons found that 47,XYY individuals were more likely to exhibit antisocial tendencies than those without this genotype. These studies erroneously assumed that an extra Y chromosome would lead to increased testosterone levels. However, follow-up studies conducted by geneticists on a general XY population found no correlation between the presence of an extra Y chromosome and behavior. In these studies, the vast majority of XYY individuals exhibited normal testosterone levels, which should not be surprising, since testosterone is a steroid (i.e., not protein-based) hormone and its levels in the body are influenced by a combined effect of many genes with a major input of genes located on the X chromosome.
Turner’s Syndrome (TS), XO Gonadal Dysgenesis (45, X)
Frequency: 1 for 2000 newborn XX individuals, with only half due to the X monosomy, while the other half resulting from other genetic factors, including chromosomal fusions, ring chromosomes and chromosomal deletions.
Turner’s syndrome is the most common karyotype among miscarriages and the only surviving monosomy of all human chromosomal aneuploids. Characteristics of TS include short stature, the lack of sexual development at puberty (underdeveloped ovaries and lack of estrogen), heart defects, kidney abnormalities, cystic neck hydromas etc.
Structural Chromosomal Abnormalities
Credit: National Human Genome Research Institute, CC BY 2.0.
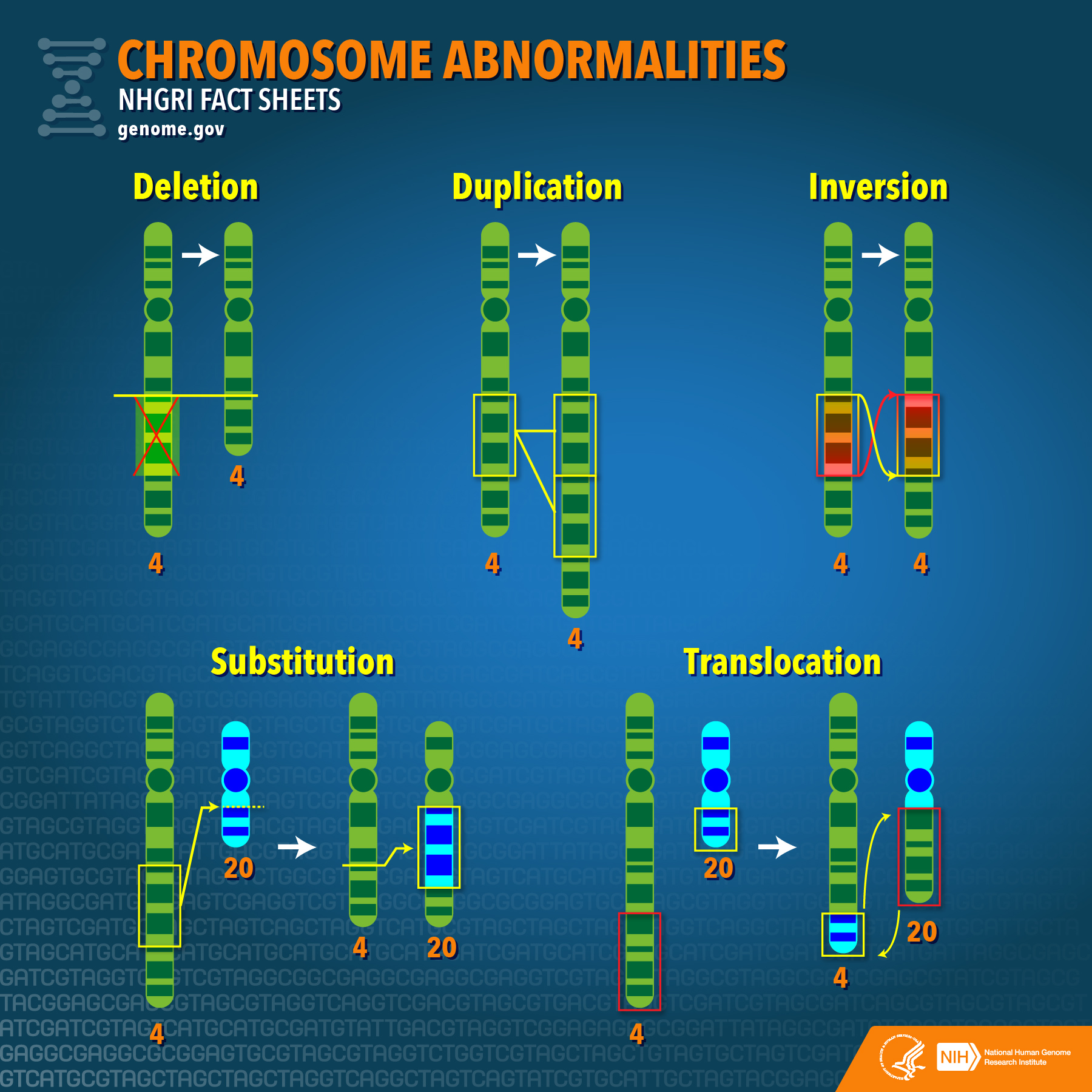
Structural chromosomal abnormalities include deletions, duplications, inversions, and translocations (substitutions, shown on the image above, are a type of a translocation).
Chromosomal deletions
Chromosomal deletion is characterized by the loss of a chromosomal segment. A number of human conditions (syndromes) occur when parts of chromosomes go missing. These conditions are usually associated with severe congenital abnormalities. Examples of human conditions resulting from deletions include Cri-Du-Chat, Williams and Prader-Willi/ Angelman syndromes.
Cri-Du-Chat Syndrome (1/50,000 live births) results from a loss of much of the short arm of chromosome 5 (5p). Symptoms include cognitive development problems.
Williams (7q11.23, 1/14,000 live births) is a neurodevelopmental disorder caused by a deletion of about 26 genes.
Prader-Willi (PWS)/ Angelman (AS) Syndrome (15q11-q13 deletion, ~1/12,500 frequency) is a neurological disorder manifesting in developmental delay, low muscle tone (in PWS), distinct personality features (happy disposition) and movement discoordination (in Angelman). While AS and PWS are clinically distinct conditions, their underlaying cause is the same chromosomal deletion. Different physiological manifestations of these disorders are a result of genomic imprinting. The 15q11-q13 deletion on the maternal chromosome leads to AS, while the same deletion on the paternal chromosome results in PWS.
Chromosomal duplications
In chromosomal duplications, extra copies of a chromosomal region are formed, resulting in different copy numbers of genes within that area of the chromosome. Chromosomes that carry a duplication contain extra copies of genetic material, which may cause the genes within a duplication not to function properly.
Example of a chromosomal duplication in humans: Charcot-Marie Tooth syndrome (CMT)
- Frequency: 1/2,500.
- Cytology: duplication of the 17p12 chromosomal region.
- Symptoms: CMT is a progressive neurological disorder. CMT patients slowly lose normal use of their feet/legs and hands/ arms as nerves to the extremities degenerate.
Chromosomal inversions
Chromosomal inversions occur when a segment breaks off and reattaches within the same chromosome. Chromosomal inversions are classified as pericentric, if the inverted fragment includes the centromere, and paracentic, if the centromere is not included. Pericentric inversions are most frequent, often reported for chromosomes 1, 2, 3, 5, 9, 10 and 16. The frequency in the general population of chromosome inversions is 1–5 in 10,000 for paracentric inversions and 1–7 in 10,000 for pericentric inversions.
Many chromosomal inversions in humans do not show a phenotypic effect. However, 45 percent of severe hemophilia A cases are due to inversions.
Chromosomal inversions can also be coupled with duplications. An example of such inverted duplications is spinal muscular atrophy (SMA), a recessive-lethal condition affecting 1/10,000 births, which, in ~18% of cases, is associated with rearrangements in an inverted duplication in 5q13.
Translocations
Translocation is a type of chromosomal abnormality in which a chromosome breaks and a portion of it reattaches to a different chromosome.
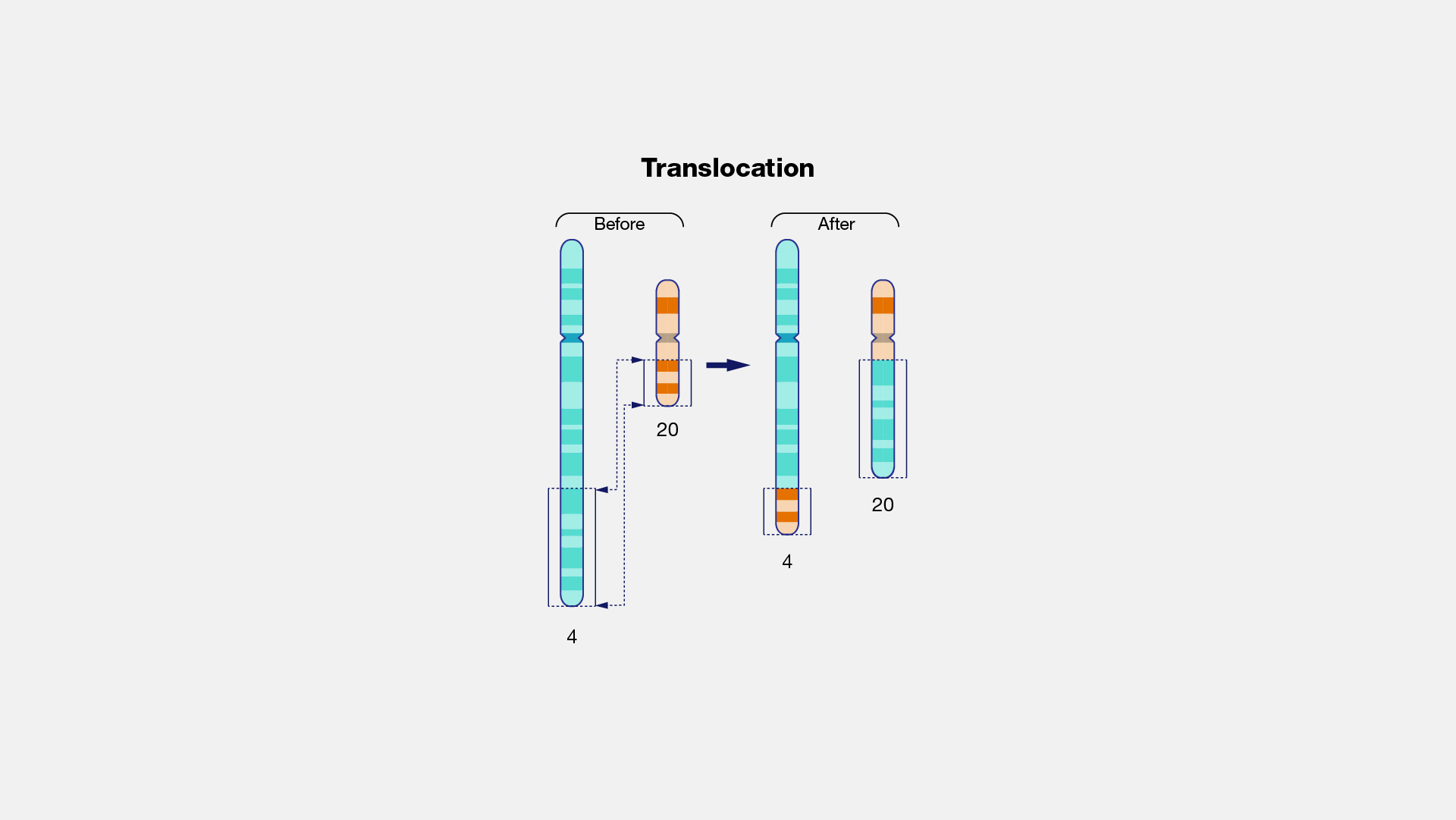
The two main types of translocations are reciprocal translocations and Robertsonian translocations.
Reciprocal translocations are an exchange of material between nonhomologous chromosomes (1/625 newborns).
In Robertsonian translocations, the participating non-homologous acrocentric chromosomes break at their centromeres and the long arms fuse to form a single, large chromosome with a single centromere. The short arms also join to form a smaller reciprocal product, which typically contains nonessential genes and is usually lost within a few cell divisions.
Chromosomal translocations are are often associated with cancer, both with respect to tumor onset and tumor progression. Recurrent translocations are commonly found in hematopoietic malignancies such as leukemia and lymphomas, as well as in solid tumors, such as certain brain tumors, prostate and lung cancers.
14q/21q Robertsonian translocation and Down syndrome
About 9% of Down syndrome children born to mothers who are less than 30 years of age are the result of Robertsonian translocation.
Robertsonian translocations are passed from generation to generation, and with this type of inheritance Down syndrome may “run in families.”
Prenatal Diagnostics
Prenatal diagnosis employs a variety of techniques to determine the health and condition of an unborn fetus. Without knowledge gained by prenatal diagnosis, there could be an untoward outcome for the fetus or the mother or both. Congenital anomalies account for 20 to 25% of perinatal deaths. Specifically, prenatal diagnosis is helpful for:
Managing the remaining weeks of the pregnancy. Determining the outcome of the pregnancy.
Planning for possible complications with the birth process. Planning for problems that may occur in the newborn infant. Deciding whether to continue the pregnancy.
Finding conditions that may affect future pregnancies.
There are a variety of non-invasive and invasive techniques available for prenatal diagnosis. Each of them can be applied only during specific time periods during the pregnancy for greatest utility. The techniques employed for prenatal diagnosis include:
- Ultrasonography
- Amniocentesis
- Chorionic villus sampling
- Fetal blood cells in maternal blood
- Maternal serum alpha-fetoprotein
- Maternal serum beta-HCG (human chorionic gonadotropin).
- Maternal serum estriol
Key Takeaways
- Chromosomal abnormalities can be structural (aneuploidy) or numerical.
- Chromosomal aneuploidy is caused by chromosomal nondisjunction during meiosis or mitosis.
- Structural chromosomal abnormalities such as translocations can be associated with cancers.
Transposable elements (transposons, mobile genetic elements) are sequences of DNA capable of mobility within a genome.
