4 Cardiac Imaging and Surgical Interventions
While exercise can be used as a tool to stress the heart by causing increases in heart rate and systolic blood pressure, a variety of tools are used to visualize aspects of the heart during these stress conditions.
Clinicians may wish to examine aspects such as:
- Motions of the cardiac muscle and chambers
- Visualization of cardiac structures, like heart valves
- Myocardial muscle tissue perfusion
- Visualizing and identifying occlusions in the coronary arteries
Additionally, medical innovations have provided a variety of interventional techniques to try and treat various heart conditions, repair heart valves, and restore blood flow to the heart. This chapter will examine some of the most common cardiac imaging techniques used with cardiac patients. Some of these imaging techniques are used in concert with an exercise stress test. It will also examine a range of common interventional techniques to assist the cardiac patient with their condition.
Cardiac Imaging
Resting and Stress Echocardiogram
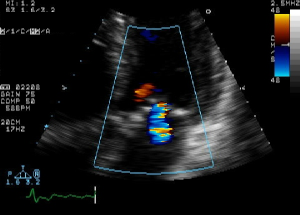
How do bats see at night? They emit clicks or other sounds that are high frequency. These soundwaves bounce off objects and there is a reflected wave they pick up with their sensitive hearing. With this information they can make an image of their surroundings. Echocardiography is similar, with a transducer wand that is placed on the chest at specific locations to specifically image the heart. High frequency sound is emitted by the wand and the waves reflect off the hard and soft structures with a receiver on the wand picking up these signals. A real-time moving image of the reflected waves is produced, showing the actions of the heart muscle, valves and the dimensions of the atrial and ventricular chambers can be assessed.
Resting Echocardiogram
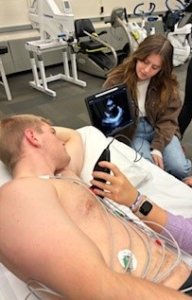
To get the clearest view of the heart, the patient is asked to lie on their left side with their left arm above their head. This allows the heart to settle closer to the side of the body and prevents the lungs and ribs from obscuring the view.
Transducers are placed at specific locations on the chest to obtain the desired images.
Views on the echo are inverted and reversed, which makes it difficult for the non-sonographer to get used to without some assistance!
In some cases, it may be necessary to obtain an echocardiogram by getting closer to the heart. This can be done by placing the transducer down the esophagus, and in some cases placed into the cardiac chamber itself.
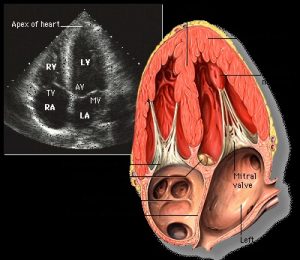
What can be assessed?- The echocardiogram is linked to the cardiac cycle. Images can be freeze-framed during the diastolic or systolic phases, and measurements taken for the chamber dimensions. Therefore End-diastolic and end-systolic volumes, stroke volume, ejection fraction can all be measured. In addition, the radiologist and cardiologist can view the contractile actions of the heart muscle, looking for areas of hypokinesis (reduced movement). These are areas where the heart muscle is not contracting as forcefully as the rest of the myocardium, possibly indicating ischemia or heart damage.
Video link
Watch this video showing the apical view on an echocardiogram.
Doppler Echo
One additional capability of the echocardiogram is to track the movement of blood through the chambers of the heart. Normally, blood flows in one direction, from atria to ventricle, and then out the ventricle into circulation. However, there can be backflow or “regurgitation” through the heart valves due to some type of valvular issue. To track blood flow, a setting on the echocardiogram known as the Doppler Echo is used.
Doppler? What is the Doppler Effect?
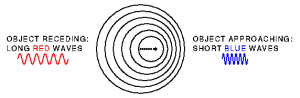
Sound waves travel at consistent frequencies when emitted from a stationary device. However, the reflection (echo) of the soundwaves back to the receiver will be influenced by the movement direction of the objects. In this case, it is the red blood cells, and they may be moving toward or away from the receiver. If objects are moving towards the receiver, the sound waves will be compressed. If they are moving away from the receiver the waves will be stretched. Astronomers could track the movement of stars in relation to the earth by measuring light frequency shifts from stars. If a star’s light had shifted to longer wavelengths (more to red color) they knew that the star was moving away for us on earth. Likewise, if the frequencies were more compressed (towards the blue) then the star would be moving towards the earth. Doppler is also used to track the movement of storms by reflecting radar off water droplets in the atmosphere.
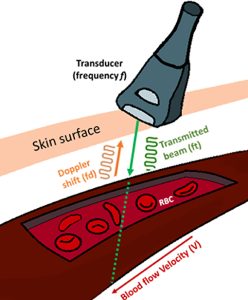
With the doppler echo, sound wave frequency differences are converted to colors, so that the direction of blood flow can be visualized, real time during the cardiac cycle. Most echo machines encode their colors so that blue represents flow away from the transducer, and red is towards the transducer (opposite of the doppler effect for visible light!)The doppler can also track blood flow through blood vessels as each ventricular pulse pushes the blood through arteries.
Nuclear Imaging: Resting and Exercise Stress Thallium Scan
While the echocardiogram can visualize the action of the heart and movement of the blood, it cannot indicate whether the heart muscle itself is receiving oxygen from the coronary arteries. For this, chemical agents are used. The chemicals are radioisotopes, and therefore emit small amounts of radiation. These emissions can serve as a marker of their location in the body when injected into the bloodstream. Thallium is a chemical that behaves like Potassium in its movement. That is, it will concentrate inside of cells if it is delivered to the area by the circulatory system.
Initial Resting scan
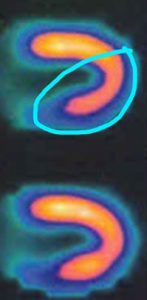
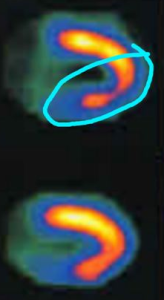
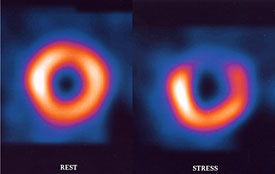
Thallium is injected into the bloodstream and the patient is allowed to rest quietly for a short period. This gives time for the Thallium to be transported into the cardiac cells. Then they are placed within a scanner (Figure 7) that can pick up the emissions from the thallium in the myocardial cells. A resting image, typically in the shape of a horseshoe or a donut display thallium emissions, and therefore blood flow to the myocardium. Brighter color indicates healthy flow.
After the resting scan is completed, an exercise stress test, or pharmacologic stress test is administered. The work of the heart (heart rate and systolic blood pressure) is increased, and near peak exercise more Thallium is injected into the bloodstream. The stress test is ended, and the patient is taken for a second scan. Resting and stress images are compared. If there are areas not receiving blood flow, there will be reduced Thallium emissions. This is known as a cold spot. If a cold spot is seen during both the resting and stress scan, then the tissue is probably necrotic (dead), possibly from a previous MI. If the resting scan looks healthy, but the stress scan has a cold spot, then it is most likely due to ischemia and an artery that is partially occluded.
|
|
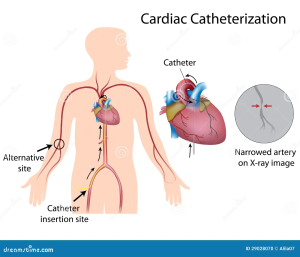
Coronary Angiography
Coronary angiography is a method used to directly visualize blood flow within the coronary arteries. This procedure uses x-rays and a radio-opaque dye, that shows up (dark color) under x-ray. The procedure begins with a catheter being introduced into the arterial system, typically either the femoral artery, brachial, or the radial artery. A wire with a flexible tube over it is guided upstream, into the aorta, until the catheter is at the entrance of the coronary arteries (just distal to the aortic valve).
Watch this animation of the angiogram procedure, courtesy of the American Heart Association.
When the catheter is in position, the dye is injected into the blood stream while an x-ray is taken. The patient is supine under the x-ray instrument, and a real-time video recording is made of the heart and coronary arteries as the dye is drawn through the arteries.
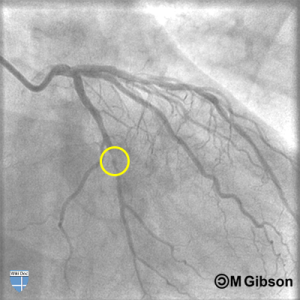
The catheter can be repositioned to inject dye into the left and right coronary arteries, providing multiple images of the arterial system. Video images can be freeze framed, and measurements of the diameter of the arteries can be taken. Occlusions appear as thin areas, where less dye is seen. These areas can be measured to determine the percentage occluded. Cardiologists can make informed decisions as to a treatment plan based on the location and degree of occlusion of one or more coronary arteries.
Flow can be graded using the TIMI system:
TIMI (Thrombolysis in myocardial Infarction) Grade Flow
TIMI 0 flow (no perfusion) refers to the absence of any antegrade flow beyond a coronary occlusion.
TIMI 1 flow (penetration without perfusion) is faint antegrade coronary flow beyond the occlusion, with incomplete filling of the distal coronary bed.
TIMI 2 flow (partial reperfusion) is delayed or sluggish antegrade flow with complete filling of the distal territory.
TIMI 3 is normal flow which fills the distal coronary bed completely.
Coronary angiography is an invaluable tool to determine the extent and location of occlusions, helping care decisions to be made with more precision. The angiography procedure is also very similar to a cardiac intervention known as angioplasty, so in emergency situations, when significant occlusion is present, angioplasty can be done at the same time as the angiogram procedure. Angioplasty will be explained in the next section.
Cardiac Interventions
Cardiac interventions can mean the difference between life and death, as well as another chance to try and reduce your risk of an adverse event. Some interventions are emergency situations, while others are planned procedures. Your cardiac patient may have had one or more of these procedures before entering rehab, so it is important to understand the procedures so you can better care for the patient.
Defibrillation
Sudden cardiac arrest is the succession of ventricular beats, and typically is accompanied by either ventricular tachycardia or ventricular fibrillation. Ventricular fibrillation especially results in random, uncoordinated firing of ventricular muscle cells, which results in a lack of systolic contraction and loss of blood flow. Death to the patient can happen within minutes. This can occur because of heart disease, heart failure, cardiomyopathy and long QT syndrome. The latter two conditions are often responsible for sudden death in athletes. Fortunately, the advent of automatic electronic defibrillators (AED’s) has resulted in a high success rate in reviving patients if they are available quickly.
Electronic defibrillation involves sending electrical energy through the chest, often 100-360 joules of energy, to spontaneously depolarize all the cardiac muscle. This can result in the SA node reestablishing a sinus beat, as all the cardiac tissue becomes ready for a sinus signal at the same time. Initial defibrillation attempts start at low energy, and if unsuccessful, the electrical energy is increased.
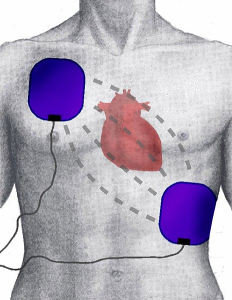
Defibrillators can be integrated with monitors that record heart rhythm, can be portable and can also be integrated within cardiac pacemakers for patients at risk of spontaneous cardiac arrest.
Cardioversion
Similar to defibrillation, cardioversion is a technique of delivering electrical energy that affects only the atrial cells. This technique is typically used in cases of atrial arrhythmias, such as atrial fibrillation, atrial flutter, some supraventricular tachycardic syndromes.
In atrial arrhythmias, the atria are sending spurious electrical signals through the AV node, but the ventricle is still performing normally. Therefore, any general defibrillation is dangerous, and could potentially send the patient into ventricular fibrillation (cardiac arrest). Therefore, the cardioversion technique uses a timed shock. That is, the shock is given at the same moment as a ventricular depolarization. This allows for the complete depolarization of the atria without impacting the ventricle (which is already depolarizing and therefore unaffected by the shock.
Chemical Cardioversion
One alternate cardioversion method involves the use of drugs instead of a shock. These drugs, such as amiodarone, propafenone, and flecainide interfere with ion channels and the depolarization of cardiac cells, reducing the excitability of the atria, increasing the opportunity for the SA node to reestablish control.
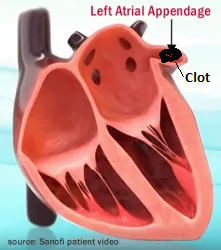
Note: Blood Clot Risk with A-Fib and Cardioversion!
In conditions of atrial fibrillation and flutter the atria is not fully contracting. This allows blood clots to form in pockets of the atria, such as the left atrial appendage. So, if a patient has been in atrial fibrillation for more than even a few hours, anti-coagulant therapy is initiated to remove any blood clots that may have formed. If not, then the contractions that would occur with cardioversion could dislodge a clot that has formed, and the patient might have a stroke.
Pacemakers
The normal cardiac cycle depends on the consistent function of the SA node to set the heart pace, followed by the transmission of the impulse through the AV node, down the bundle branches, and across the ventricles. However, there are several conditions of the heart where SA node firing is disrupted, AV node conduction is impaired, and ventricular stimulation is delayed or impaired. A pacemaker is a technology designed to help restore the normal cardiac cycle action by circumventing the limitations in the heart’s pacing system.
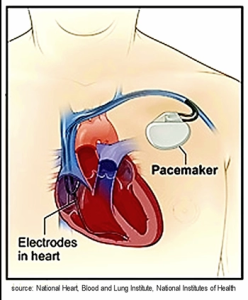
Watch this animation of a pacemaker. Courtesy of the American Heart Association.
The pacemaker can be configured to both sense heart rhythm and pace heart rhythm. It also can stimulate only the atria (“single chamber”) or can take over pacing entirely by pacing the atria, setting a delay, and then pacing the ventricle (dual chamber pacemaker). Its function is tailored to meet the requirements of the heart pacing problem.
Pacemaker beats and the electrocardiogram
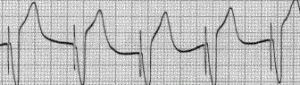
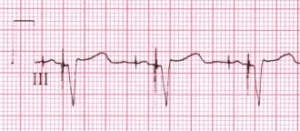
Pacemakers are organized in their function according to a series of codes that indicate which chambers are paced, which chambers (if any) are sensed, the response to the sensing and any variations in how the chambers are paced.
The revised NASPE/BPEG generic code for anti-bradycardia pacing
| I | II | III | IV | V |
| Chamber(s) paced | Chamber(s) sensed | Response to sensing | Rate modulation | Multisite pacing |
| O = None | O = None | O = None | O = None | O = None |
| A = Atrium | A = Atrium | T = Triggered | R = Rate modulation | A = Atrium |
| V = Ventricle | V = Ventricle | I = Inhibited | V = Ventricle | |
| D = Dual (A+V) | D = Dual (A+V) | D = Dual (T+I) | D = Dual (A+V) |
Table Source: https://en.ecgpedia.org/index.php?title=Pacemaker.
Examples of pacemaker configurations:
AAI: Chamber paced is A. Chamber sensed is A. Response to sensing is I. This would be used in cases of significant bradycardic rhythms. Perhaps while sleeping at night the patient’s heart rate gets exceedingly low, and the pacemaker will prevent the rate from dropping below a pre-determined rate.
VVI: Chamber paced is V. Chamber sensed is V. Response to sensing is I. The ventricles are paced, when the ventricular rhythm falls below the pacemaker’s threshold. Perhaps the patient has an AV heart block condition that may result in a very low ventricular rate.
DDD: The pacemaker sense both atrial and ventricular rates and can pace either chamber when needed.
DDDR: As above, but the pacemaker has a sensor that records a demand for higher cardiac output and can adjust the heart rate accordingly. This is helpful for exercise and other physical activity.
ICD- Internal Cardiac Defibrillators- these look like pacemakers and can sense when the patient goes into ventricular tachycardia or ventricular fibrillation. It delivers a defibrillating shock to the heart to revive the patient. Its function is exclusive to this use.
Watch this animation of an ICD device in action. Courtesy of the American Heart Association.
Precautions
Because pacemakers are controlled and coded using magnets, and provide electrical inputs to the heart, other devices can interfere with their function. The National Heart Lung and Blood Institute provides the following precautions for individuals with pacemakers:
To be safe, keep your pacemaker at least 6 inches away from such devices or only use them briefly, when needed.
Cell phones. Use your speaker phone setting or hold the cell phone to the ear on the opposite side of your body. For example, if you have an ICD on the left side of your chest, hold your cell phone to your right ear. Avoid putting your cell phone in your shirt pocket.
Headphones. Most headphones have a magnet in them. Wear them as far away from your pacemaker as possible. Do not carry your headphones in your chest pocket.
Household appliances, such as microwave ovens, major appliances, electric blankets, and heating pads are usually safe if they are working properly.
Metal detectors, such as those used for airport security. The risk of harm is low, but your device may set off the metal detector. Body scanners used at airports appear to be safe for people with pacemakers, but you can show your ID card and ask for a separate screening.
If something disrupts your pacemaker, step away from whatever is disturbing it to help your pacemaker return to normal. Talk to your doctor right away about what else to avoid, as any kind of powerful electrical or industrial equipment can interfere with your pacemaker. This includes welding machines or electric fences for pets.
Medical and dental procedures that can affect your pacemaker include:
Electrocautery used during surgery to stop blood vessels from bleeding
Magnetic resonance imaging (MRI)
Microwave diathermy for physical therapy
Radiation therapy to treat cancer
Shock-wave lithotripsy to treat kidney stones
Transcutaneous electrical nerve stimulation (TENS) to treat pain
(source: national Heart, Lung, and Blood Institute, https://www.nhlbi.nih.gov/health/pacemakers/living-with)
Percutaneous Coronary Intervention: Angioplasty
Occlusions within a coronary artery are typically in many locations. Some may not be significant enough for any surgical intervention, but others may have significant occlusions (>70%) and are reducing blood flow. The use of catheters to enter the body and gain access to the coronary arteries is a minimally invasive (artery access) procedure utilizing small catheters that can be introduced into the femoral or radial artery and be moved upstream, past the aorta and into the coronary arteries. Significant occlusions that have been identified by x-ray using angiography can be widened by inflating a balloon, crushing the plaque into the arterial wall.
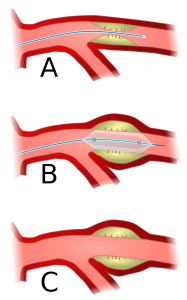
Watch this animation of balloon angioplasty. Courtesy of the American Heart Association.
The PCI procedure was introduced in the 1970’s and was successful in restoring blood flow. However, there were limitations. Inflation of the balloon can cause damage to the vascular wall, and crushed plaque initially did not have a high success rate in staying put. Plaques could rupture and reocclude, or perhaps move downstream and cause an infarction. In the 1990’s a thin metal sheath called a stent was introduced. This metal framework was pushed into place on top of the plaque that had been crushed and helps to hold the plaque in place. The incidence of re-occlusion was greatly reduced using stents. Modern stents are also coated with drugs (“drug eluting stents” that help reduce the incidence of blood clot formation and re-occlusion.
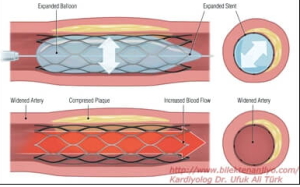
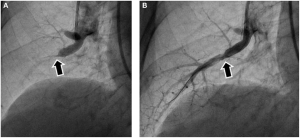
Watch this animation of the stent placement procedure. Courtesy of the American Heart Association.
Catheter Ablation to Treat Atrial Fibrillation
A third procedure that utilizes catheter access to the heart is for the treatment of atrial fibrillation. Atrial fibrillation begins as excited cells near the pulmonary veins initiating a spread of random firings in atrial cells. This chaos will spread across the entire atria and become a permanent condition if it cannot be checked. It is analogous to an out-of-control forest fire that will spread across a forest, consuming all in its path.

One solution is like firefighters creating a burn line to stop a fire. That is, they get ahead of the fire, burn a line of forest to consume the fuel for the fire and block it from spreading. In catheter ablation, tissue just beyond the pulmonary veins are ablated (burned or frozen), killing the tissue and preventing the conduction of the chaotic firing. This can prevent the rest of the atria from falling under the influence of the fibrillating tissue. Risk: Since other nerves (phrenic nerve) and tissue are near the ablation, they might also get ablated! So, the procedure needs to be deep enough to block the signals but superficial enough to not go all the way through the tissue.
In catheter ablation, a catheter is sent into the venous system (most often the femoral vein). The catheter is fed back to the heart, into the right atria. Since the pulmonary veins are in the left atria, the interatrial septum must be punctured so that the ablation tool can gain access to the location. Additional tools are also introduced into the left atria, such as an atrial mapping catheter electrode and cardiac ultrasound. Once the location of the fibrillating cells is mapped, the ablation catheter is used to kills the cells surrounding the errant tissue, isolating them from the rest of the atria.
AV Node ablation
In cases where the fibrillation is beyond the scope of pulmonary vein isolation, it may be desirable to ablate the AV node to prevent rapid atrial signaling to the ventricles. AV node ablation will require that the patient have a pacemaker installed to take over the regulation of heart pacing.
Maze Procedure
An additional attempt to control atrial fibrillation is to use a heated scalpel to cut a series of lines across the atria to better control the fibrillating tissue. The “maze” of scar tissue can help control fibrillation and help restore heart rate control. Since this procedure is done by opening the chest, it is often paired with other open-heart procedures (i.e. valve repair, CABG surgery).
New Ablation Procedure : FARAPULSE
In 2024 the FDA approved a new type of procedure that uses pulses of electrical waves instead of heat or cold to ablate the cardiac tissue. A catheter that spreads out like a flower, contacting tissue around the pulmonary veins and deploying an electric field. The advantage of this procedure is that the ablation does not penetrate the tissue, so the nerves and deeper tissue are safe from damage.
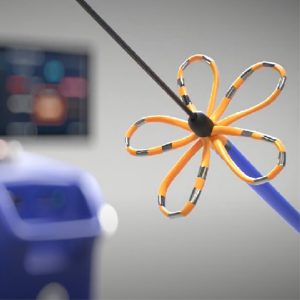
Watch this video of the FARAPULSE procedure.
Isolation of Left Atrial Appendage: Watchman Procedure to treat Atrial Fibrillation
One other procedure related to atrial fibrillation involves the clot forming area of the left atrial appendage. If the atrial fibrillation is chronic, then blood clot formation in this area is a major risk. One technique known as the Watchman procedure is designed to close the appendage and prevent blood from pooling.

Valve Repair
There are four heart valves that need to both open completely to allow blood flow, and then close tight to prevent the backwards flow (regurgitation) of blood in the wrong direction. Valves can become calcified and have a reduction in the ability to open (stenotic valve) or the valve regurgitates blood backwards. The most common heart valve problem happens to the left side of the heart with the aortic and mitral valve. Mitral valve regurgitation and aortic stenosis being the most common. Previously, repair or replacement of a valve was done with open heart surgery. While this still may be the case in some instances, more often a catheter method is used to repair the valve annulus, leaflet, or simply replace the valve.
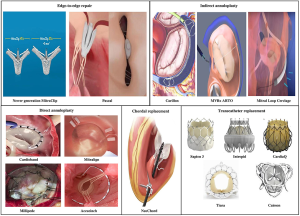
For patients receiving percutaneous (catheter-based) heart valve repair or replacement, there will be minimal post-surgical wound issues. However, the exercise science professional will need to understand their overall health status, since heart valve problems are often linked with other cardiac concerns (heart failure, hypertension) so each patient will need to be assessed and a risk profile created.
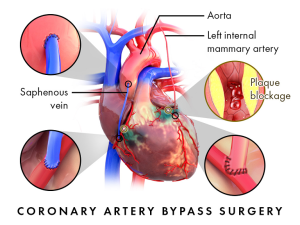
Coronary Artery Bypass Graft Surgery (CABG)
The most invasive among the heart interventions was first developed in the 1960’s to circumvent occlusions in the artery. There was no angioplasty procedure yet, so surgeons devised a method to use other blood vessels within the body to serve as another coronary artery (of sorts) to go around the occlusion (“Bypass”) and deliver blood to the heart tissue. Ideally, stealing an artery from another part of the body to use as a bypass vessel is ideal. Arteries are resilient, strong-walled and resist atherosclerosis better than veins. But you can’t steal an artery from just anywhere! So, there are only a few arteries that can be used. Likewise, veins, which help return deoxygenated blood to the heart, can only be taken from areas where alternate venous return is possible.
Steps in the Surgical Procedure
- Identifying areas of occlusion
Typically, coronary angiography or other imaging scan (such as CT scan) is used to identify the specific location of occlusions, their degree of severity as well as their anatomical location. The surgeon can plan a strategy for proceeding with the surgery, which accessory blood vessels to harvest and any other aspects of the surgery unique to the case.
- General anesthesia
The patient is placed under general anesthesia and is intubated for breathing support. The body is covered in an antiseptic solution. When the team is ready the surgery begins at the sternal site as well as any site for vessel harvesting.
Note for the exercise physiologist: Post surgical recovery from anesthesia can vary with patients. During the first few days of recovery, they still may feel some influence of the medications. Always be aware of their movement and balance when you assist them in movement!
- Sternal incision and cutting
The initial sternal incision is through the skin with a scalpel, and then the tissue is cauterized (burned) using a cauterizing knife. The knife can cut through tissue as it cauterizes and stops any bleeding. When the sternal bone is exposed, a sternal saw is used to cut through the length of the sternum (sternotomy). Retractors are placed on the sides of the sternal borders and the chest is opened so that the various tissue membranes can be penetrated until the heart is visible.
Note for the exercise physiologist: This procedure becomes a post-surgical injury that must be navigated when prescribing exercise. With a sternum cut, upper body exercise is something to be avoided until the sternum has had a chance to heal. Post surgical muscle pain, chest discomfort from the ribs being spread will be common.

- Harvesting the arteries and vein used for the procedure
Once the sternum is retracted, harvesting of blood vessels to be used in bypass surgery can begin. These vessels may include:
Left Internal Mammary Artery– This is an artery that branches off the subclavian artery. What is unique about using this artery is that one end is already attached to the arterial system, the surgeon only needs to detach the distal end of the mammary artery to move over to a location just beyond the occlusion in the coronary artery. First, the artery must be freed from the tissue and accessory blood vessels connected to it. Typically, some tissue is left with the artery to help keep the vessel healthy after the procedure. The internal mammary is often used for the most significant occlusion because of its resilience as a bypass vessel.
Radial Artery– The radial artery may also be used, most often from the non-dominant arm. In this case a section of the vessel must be detached at both ends to be used. Once the vessel is harvested it may be used in one or more bypasses depending on the lengths needed for the procedure
Saphenous Vein– The saphenous vein is a long vessel, running the length of the leg from groin to ankle. This is advantageous, since it can be used in sections for multiple bypass segments. For patients needing 3 or more bypasses, this vessel is important to utilize. Note- when the veins are used, they must be turned inside out to prevent venous valve interference with flow.
Methods of Harvesting– There are two methods of gaining access and harvesting the blood vessels. One is open surgery. This is when an incision is made along the length of the vessel to gain access and remove the blood vessel. Open surgery is used with the internal mammary artery since the chest is already open. The method may or may not be used with radial or saphenous vessels. Another harvesting technique is to use an endoscope. In this procedure a small incision is made in the tissue at one end of the blood vessel. The endoscope is fitted with a camera and tools to free the vessel from tissue and other blood vessels and detach both ends. The vessel is then removed through the small incision.
Note for the exercise physiologist: You will want to make note of the procedure used with your patient. Do they have a radial artery removed? There may be limitations in that arm following surgery. What about the saphenous vein? Was it removed? Removed endoscopically, or do they have a healing wound the length of their leg. Be aware of the status of your patient!
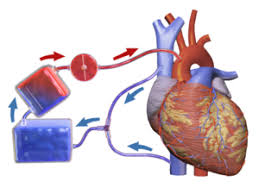
- Stopping the heart
To complete a coronary artery bypass graft, the heart must be drained of blood and stopped from beating. This of course necessitates a system to both breathe for the patient as well as pump blood. A heart-lung bypass machine is employed prior to attaching the bypass vessels. First, large tubes (called Cannulas) are placed in the vena cava or the right atria. This collects all venous blood returning to the heart. The blood is redirected through the cannulas to a reservoir, which sends the blood into an oxygenator, which perfuses the blood with oxygen. The freshly oxygenated blood is sent through another cannula that is attached to the aorta. (see figure 27)
At the same time, a solution of cold potassium chloride (“cardioplegia“) is infused into the heart to stop it from beating. The cold solution also keeps the heart at a low temperature to minimize the oxygen need of the myocardia cells.
The heart-lung bypass machine is used to maintain arterial pressure to ensure that oxygenated blood is distributed to the brain and throughout the body. This condition is maintained across much of the 3-6 hour procedure.
Attaching the bypass vessels
To bypass the occlusion in the coronary artery the accessory vessels need one end connected to the arterial system, and the other end connected to the coronary artery just distal, or just past, the occlusion. For the internal mammary artery, one end is already connected to the arterial system, so it is left connected. The other end is brought to the area of occlusion. A hole is punched in the coronary artery and the end of the mammary artery is stitched or “grafted” to the hole into coronary artery (called and “end to side anastomosis”). This work is done manually by the surgeon, and a tight connection is needed so that there are no leaks. For the radial artery or saphenous vein, both ends must be connected. One end is stitched to a hole placed in the aorta just past the aortic valve and the other to a hole punched distal to the occlusion in the coronary artery (see figure 28).
Restarting the heart and closing the chest
Once the bypass grafts are in place, they begin to warm the heart and clear the KCL cardioplegia solution from the system. Then the patient is removed from the heart-lung bypass machine, cannulas removed, and holes stitched. This is a critical juncture where the heart needs to be warm enough to begin to beat on its own. Often defibrillation is required to try and restart the heart. Once the heart is restarted, the surgeon can check all the bypass grafts for any leaks and a final assessment of the work is done.
Closure of the chest is done by removing the chest retractors and placing wire in the sternum on both sides of the incision, the wires are used to pull the chest/sternum together. Glue may also be used. The wires are twisted and held secure and then cut to fit under the skin. Final steps include pulling the skin and gluing the skin together or possibly using skin staples.
Note for the exercise physiologist: think of the wounds and pain the patient will have after this surgery. They may be missing a vein (saphenous) and could have a long incision to monitor on the leg, or arm (radial artery site). They will have chest pain from the sternal incision, which will take weeks to heal. Be sure to consider the limitations due to the surgery along with their cardiac condition when working with them on their physical recovery.
Heart Failure Treatment- The “LVAD”
In the heart failure patient, there is a progressive loss in ejection fraction, leading to a significant reduction in cardiac output. This can also limit the blood flow return to the coronary arteries. With severely compromised patients, a heart transplant may be the last option. Unfortunately, the number of heart transplants in the US has not changed significantly since 1990. In that year, 2,107 heart transplants were performed, compared to 3,817 in 2021. According to the Journal of Cardiac Failure, 6.7 million Americans over age 20 have heart failure, and is expected to rise to 8.5 million by 2030 .
Clearly heart transplants cannot meet the need of the severe heart failure patient. To that end, a mechanical device has been developed to assist the left ventricle with the pumping of blood through systemic circulation. Called a Left Ventricular Assist Device, or LVAD, it is a device that funnels blood out of the left ventricle and then sends it via pump system into the aorta.
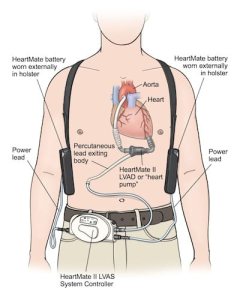
The LVAD is used a bridge for those awaiting heart transplant but can also be used long term for patients that are not eligible for transplant (termed “destination therapy”, or until death). The LVAD significantly increases cardiac output for the patient, to the point where they may see a significant increase in work capacity and quality of life. LVAD patients can also participate in cardiac rehab to strengthen their heart to reduce reliance on the LVAD and also improve systemic circulation and coronary flow.
Improving coronary blood flow without exercise: Externally Enhanced Counter Pulsation Therapy
You learned in earlier chapters that coronary blood flow happens primarily during diastole, when the ventricle relaxes and blood flow in the aorta is drawn back, closing the aortic valve, and creating a pressure wave that pushes blood into the coronary arteries.
However, in some patients (i.e. heart failure) there is very little pressure pushing blood into the coronary arteries. These patients may not be able to tolerate exercise, so a mechanical means of perfusing the coronary arteries has been developed.
In this therapy, the patient has their lower limbs wrapped in a series of inflatable cuffs (like a blood pressure cuff). They are also fitted to an ECG so that systolic and diastolic phases of heart action can be identified. The ECG and pressurization of the cuffs are sequenced, such that during the diastolic phase, the cuffs inflate rapidly (>200mmHg!), pushing blood towards the heart, creating a pressure wave that sends oxygenated blood from the aorta into the coronary arteries. The cuff then rapidly deflates during systole so that the ventricle can send blood out of the heart. This timed diastolic pressure treatment takes place for 20-30 minutes each session.
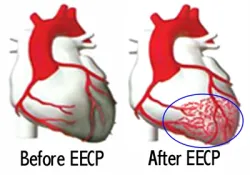
This chronic pressurization and increased blood flow into the coronary arteries has been shown to increase not only coronary blood flow, but also stimulate the growth of new, collateral blood vessels. This improves blood flow to larger portions of the heart muscle, increasing oxygen availability to the tissue.
Selected Sources
Handbook of Cardiac Anatomy, Physiology and Devices. 3rd ed. Iaizzo P. editor. Springer pub. 2015.
AACVPR Cardiac Rehabilitation Resource Manual. American Association of Cardiovascular and Pulmonary Rehabilitation. Human Kinetics Pub. 2006.
Clinical Exercise Electrocardiography. Levine S., Coyne B., Colvin L. Jones & Bartlett Learning Pub. 2016.
Number of heart transplantations in the U.S. from 1975 to 2021. Statista.com https://www.statista.com/statistics/671451/heart-transplants-number-us/#:~:text=Heart%20transplants%20in%20the%20U.S.%201975%2D2021&text=In%202021%2C%20there%20were%20approximately,U.S.%20from%201975%20to%202021.
Heart Failure Epidemiology and Outcomes Statistics: A Report of the Heart Failure Society of America. Bozkurt B. et.al. VOLUME 29, ISSUE 10, P1412-1451, OCTOBER 2023
Video links used in Chapter 4
Apical View on Echocardiogram: https://youtu.be/lpcJdTwmLRw
Angiogram Procedure: https://watchlearnlive.heart.org/?moduleSelect=angiog
Pacemaker Animation: https://watchlearnlive.heart.org/?moduleSelect=pacmkr
Internal Cardiac Defibrillator: https://watchlearnlive.heart.org/?moduleSelect=icddev
Animation of Balloon Angioplasty: https://watchlearnlive.heart.org/?moduleSelect=angiop
Stent Placement: https://watchlearnlive.heart.org/?moduleSelect=cstent
The process of delivering blood, oxygen and nutrients to the tissue and organs. In heart disease, arteries that are partially occluded can result in decrease perfusion to tissue.
The volume in the ventricle at the end of systole. It is influenced by both preload and afterload as well as the strength of heart contraction.
The volume of blood ejected from the left ventricle with each contraction.
The percentage of blood ejected from the left ventricle relative to the end diastolic volume. It is a key measure of heart contraction strength. EF= (stroke volume/ EDV) x 100
A reduction in normal movement or contraction. In cardiac muscle this is reduce contractile action of the cardiac chambers, due to ischemia or necrotic (dead) tissue)
The abnormal backflow of a fluid. With respect to a heart valve, blood typically flows in one direction through an open valve and then when the valve closes, flow cannot go back. However impaired valves can create openings allowing for blood to flow backwards. Mitral valve prolapse is a condition where regurgitation occurs.
Referring to dead tissue. Necrotic tissue is no longer viable.
Inadequate blood (and therefore oxygen) supply to a tissue. A narrowed or blocked coronary artery results in reduced blood flow to an area of the heart, resulting in reduced oxygen availability.
Thrombolysis In Myocardial Infarction. A grading system of flow, where 0= no flow and 3 = normal flow
Percutaneous Coronary Intervention. A procedure where a catheter equipped with a balloon is sent upstream on the arterial side (femoral or radial) to an occluded area in a coronary artery. The balloon is inflated, and the area is widened.
a device that sends an electric shock through the heart, depolarizing all cells at once, with the hope that the SA node resumes its role as the normal pacemaker. Defibrillation can be done externally to the chest, and internal defibrillators can be implanted as part of a pacemaker.
An abnormality of the heart in rhythm or rate. Synonymous with dysrhythmia
A timed defibrillation procedure where the depolarization shock is synchronized with the ventricular systole to prevent inducing dangerous arrhythmias. It is used in cases of atrial fibrillation, flutter and SVT. Chemical cardioversion is also possible with medications such as flecainide, amiodarone or propafenone.
Percutaneous Coronary Intervention. A procedure where a catheter equipped with a balloon is sent upstream on the arterial side (femoral or radial) to an occluded area in a coronary artery. The balloon is inflated, and the area is widened.
A small, metal mesh-like tube that is placed in the coronary artery to keep a blood vessel open after a PCI or angioplasty procedure. Typically, it is put in place following balloon angioplasty has widened a narrowed area in the artery.
Procedure to destroy tissue that is abnormal or malfunctioning. In cardiac procedures it is often a catheter procedure, using heat, cold or electrical energy. Common to atrial fibrillation treatment.
A surgical procedure involving the cutting of the sternum lengthwise to gain access to the heart and surrounding tissue.
Thin tubes that are inserted into the body to administer fluids or redirect fluids, such as is the case with a heart lung bypass machine.
A technique of administration of a solution containing potassium, magnesium, and calcium to stop the heart by eliminating voltage gradients across cell membranes. This is used during open heart surgery so that the surgeon can operate on a still heart.
A surgical connection between two structures, such as blood vessels. In open heart surgery these are manual stitched connections by the surgeon in order to reroute blood flow.
